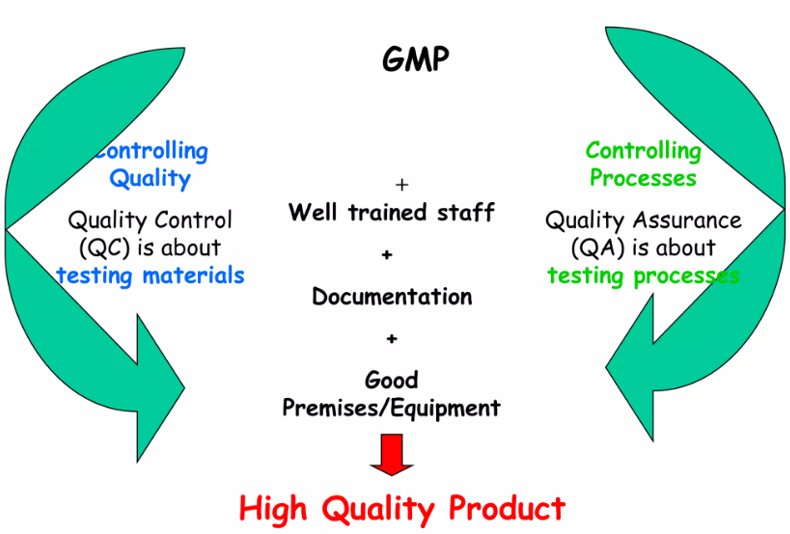
Good manufacturing practice (GMP) is that part of a quality management system to ensure that products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorization.
Why GMP?
- GMP is aimed primarily at diminishing the risks inherent in any pharmaceutical production;
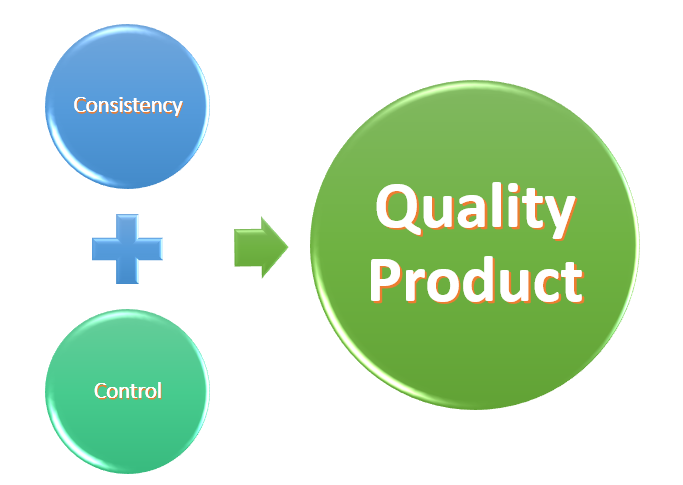
- Adopt GMP to achieve a quality product we must Build Quality into our systems and processes.
- GMP provide a basic assurance that a product was produced under industry-standard conditions.
Some of the areas addressed in GMPs include:
- Building & facility conditions
- Equipment design and maintenance
- Employee practices
- Sanitation conditions
- Raw ingredient sourcing
- Maintaining strong production controls
- Records and reports
There are several sets of GMP standards which have been endorsed by different governments. Fortunately, although they are nearly identical. Some versions of GMPs include: CGMP (USA), EU-GMP, Guide 104 GMP (Canada), PIC/s, JAPAN-GMP, WHO-GMP
GMPc Vietnam is recognized throughout Vietnam as the leader in providing turnkey consulting solutions for GMP-certified facility projects, including Pharmaceuticals, Cosmetics, Health supplements and Veterinary pharmaceutical. Though 12 years of development 2011 to 2023, GMPc has implemented more than 230 GMP-certified facility projects, equaling to more than 80% of market share of the field in Vietnam. Not only do domestic customers, foreign investors also choose GMPc as their consultant when investing new factories in Vietnam, such as Kyoto Biken Vaccine Factory, Nippon Chemiphar Pharmaceutical Factory, Shimizu Contractor, Kajima Contractor, etc.
Consultant services by GMPc Vietnam
GMP project consulted by GMPc Vietnam


